4.5 out of 5 | 800+ reviews
Put in



Wrinkle cream that actually works
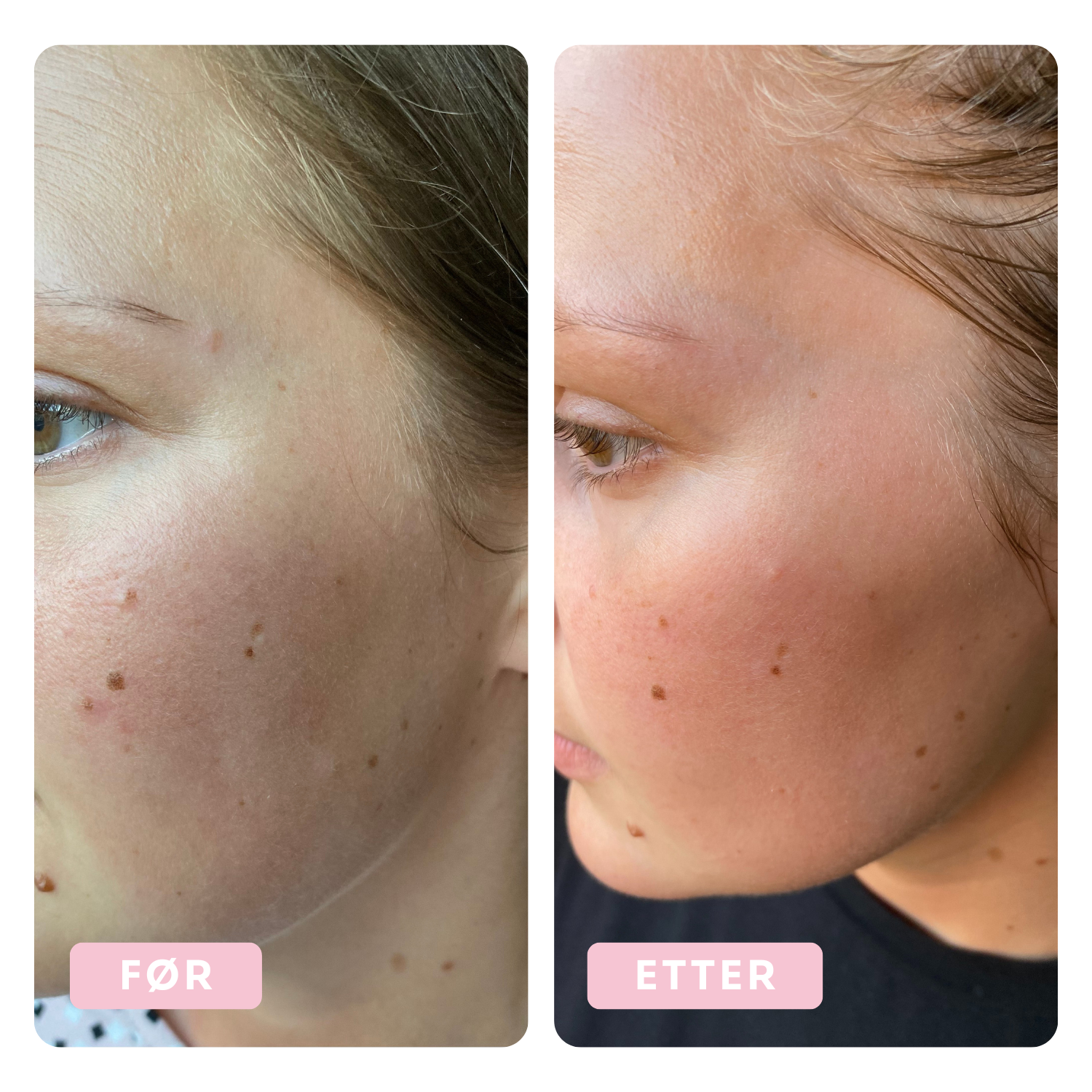
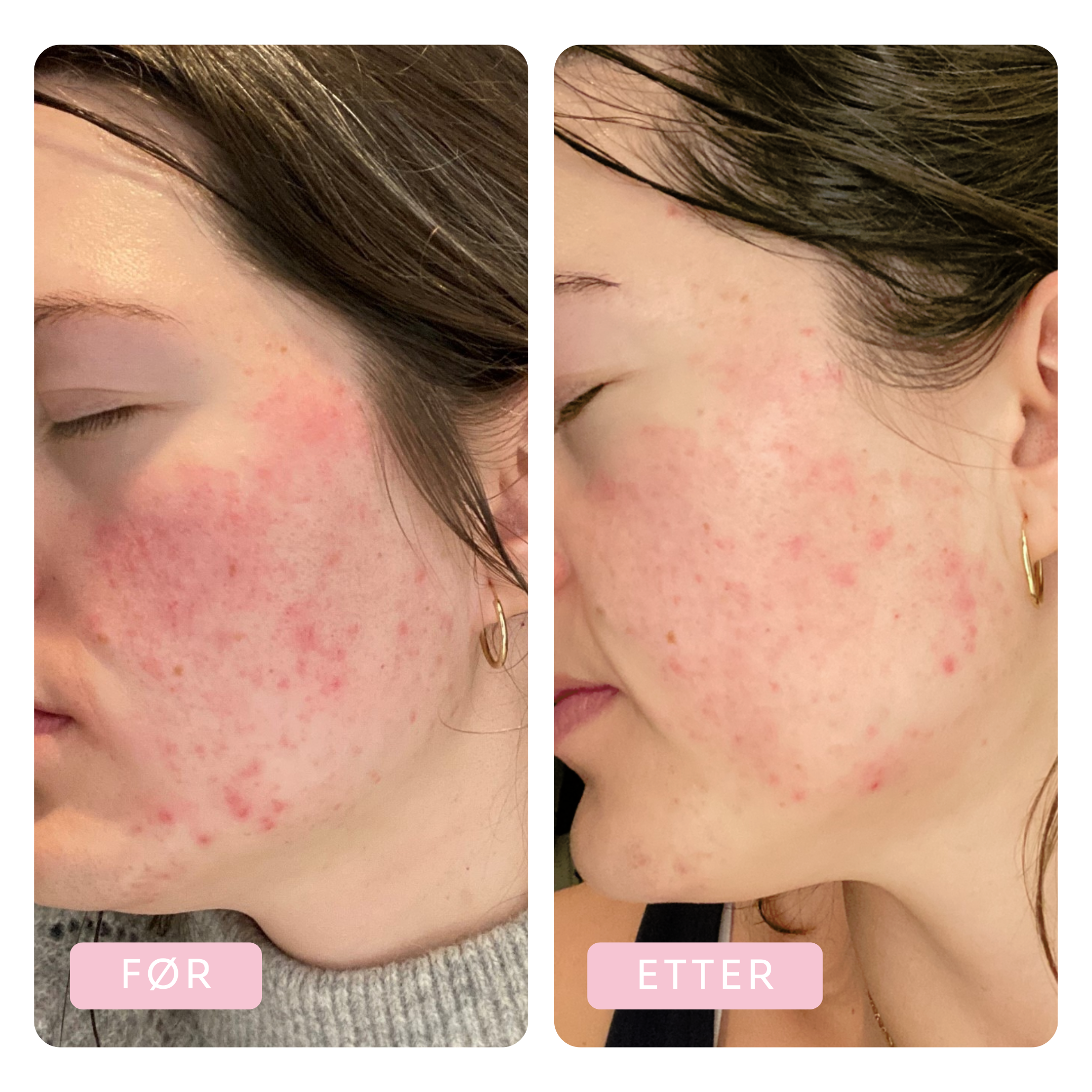
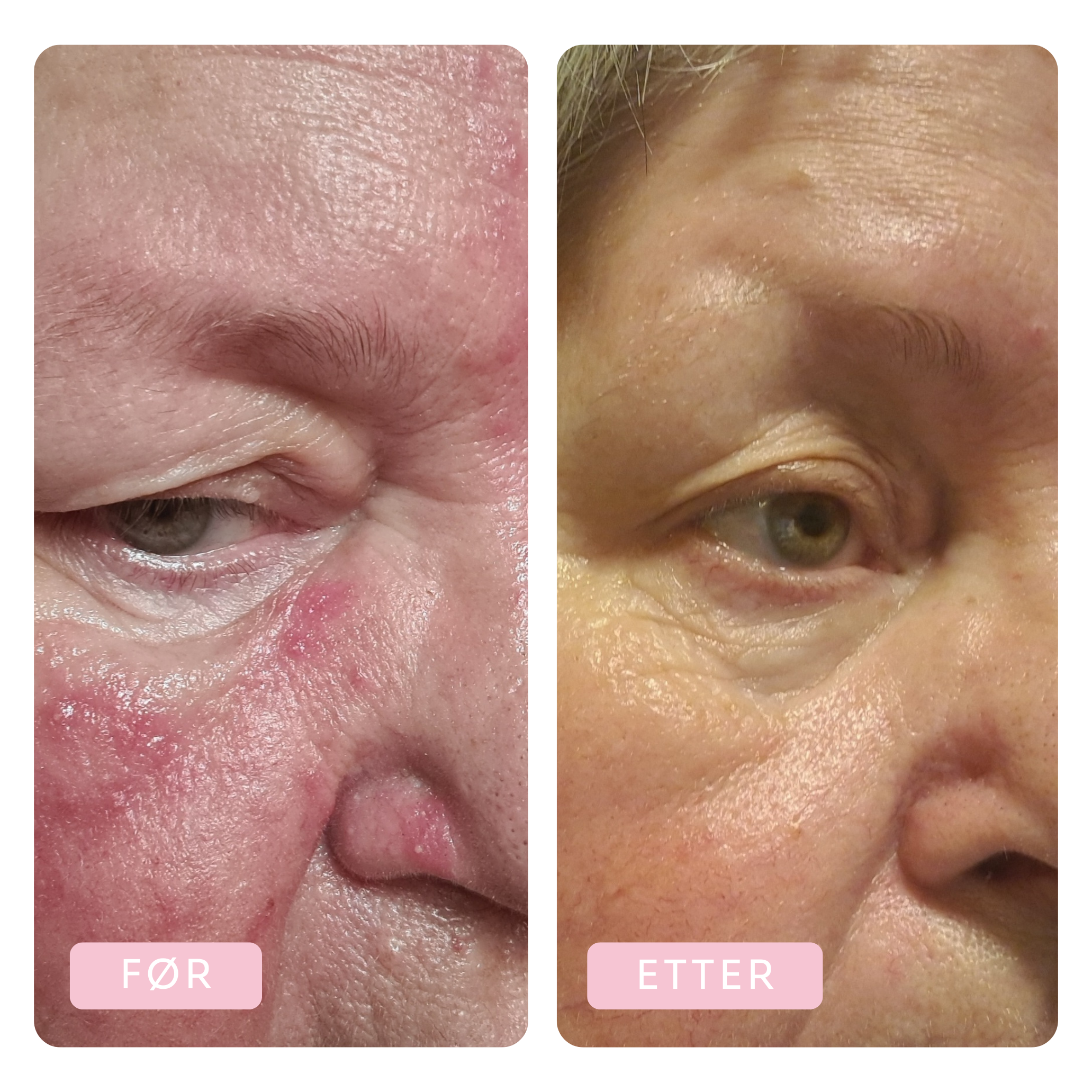
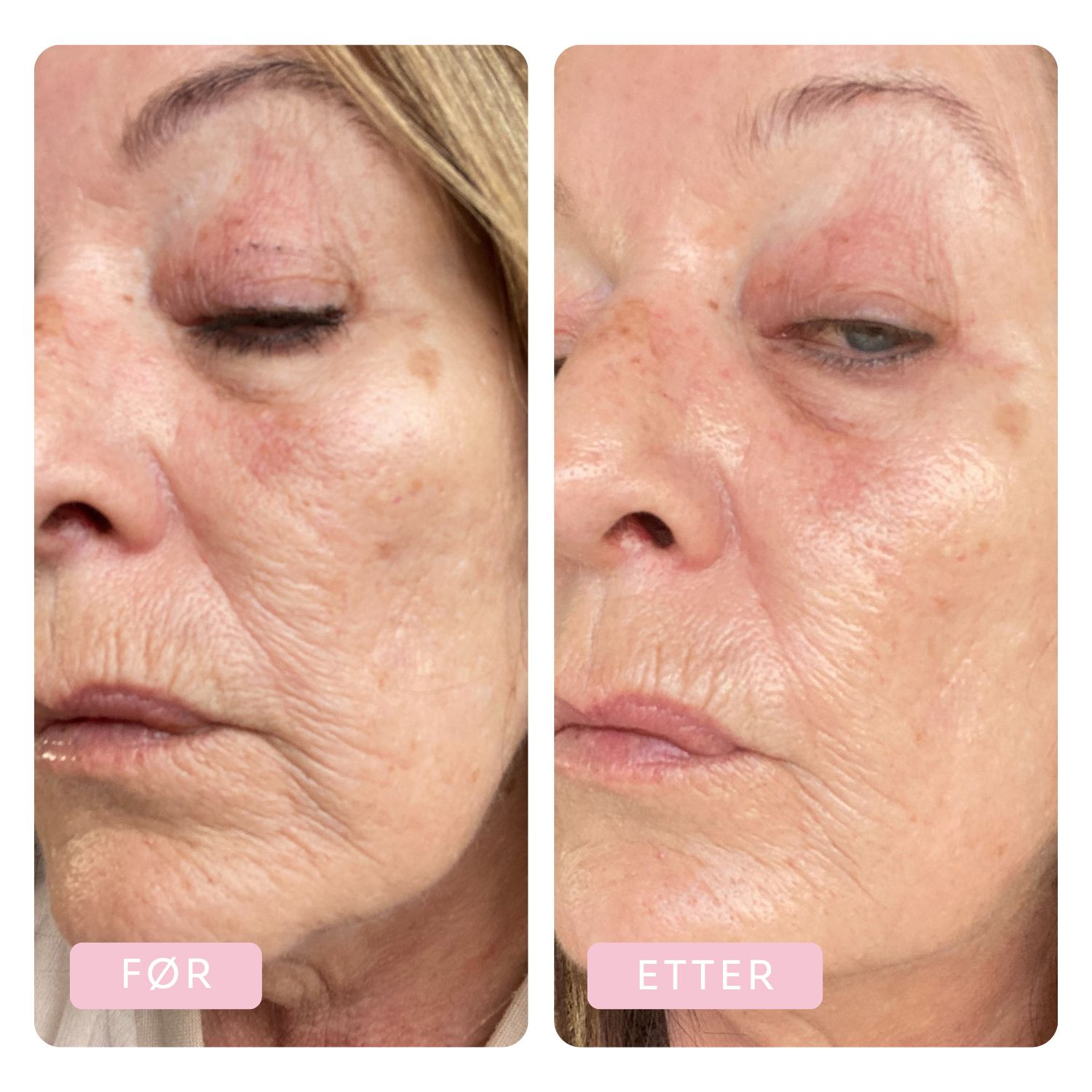
Before & After
Tora has been using the Dr.Stine THE set for 6 weeks
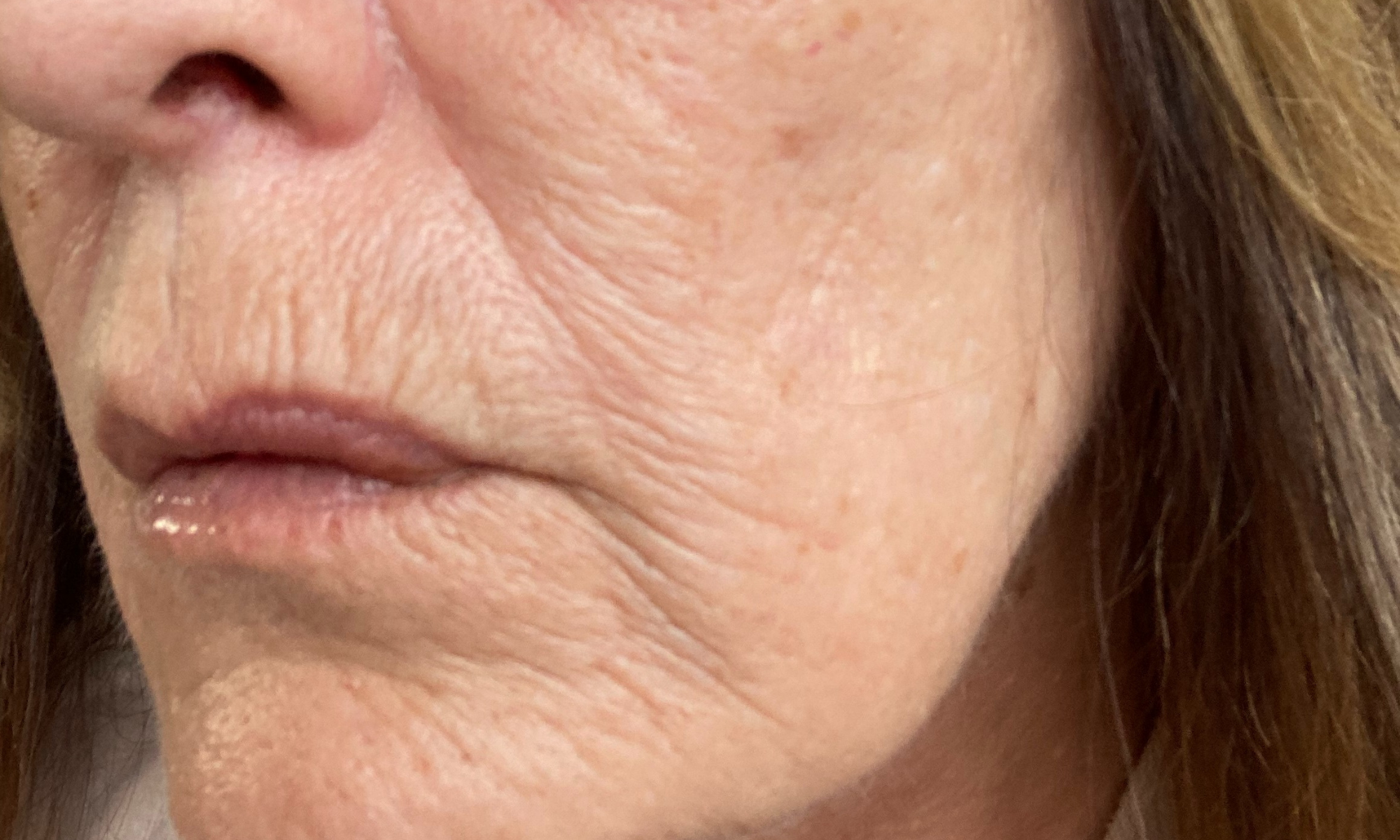
Before
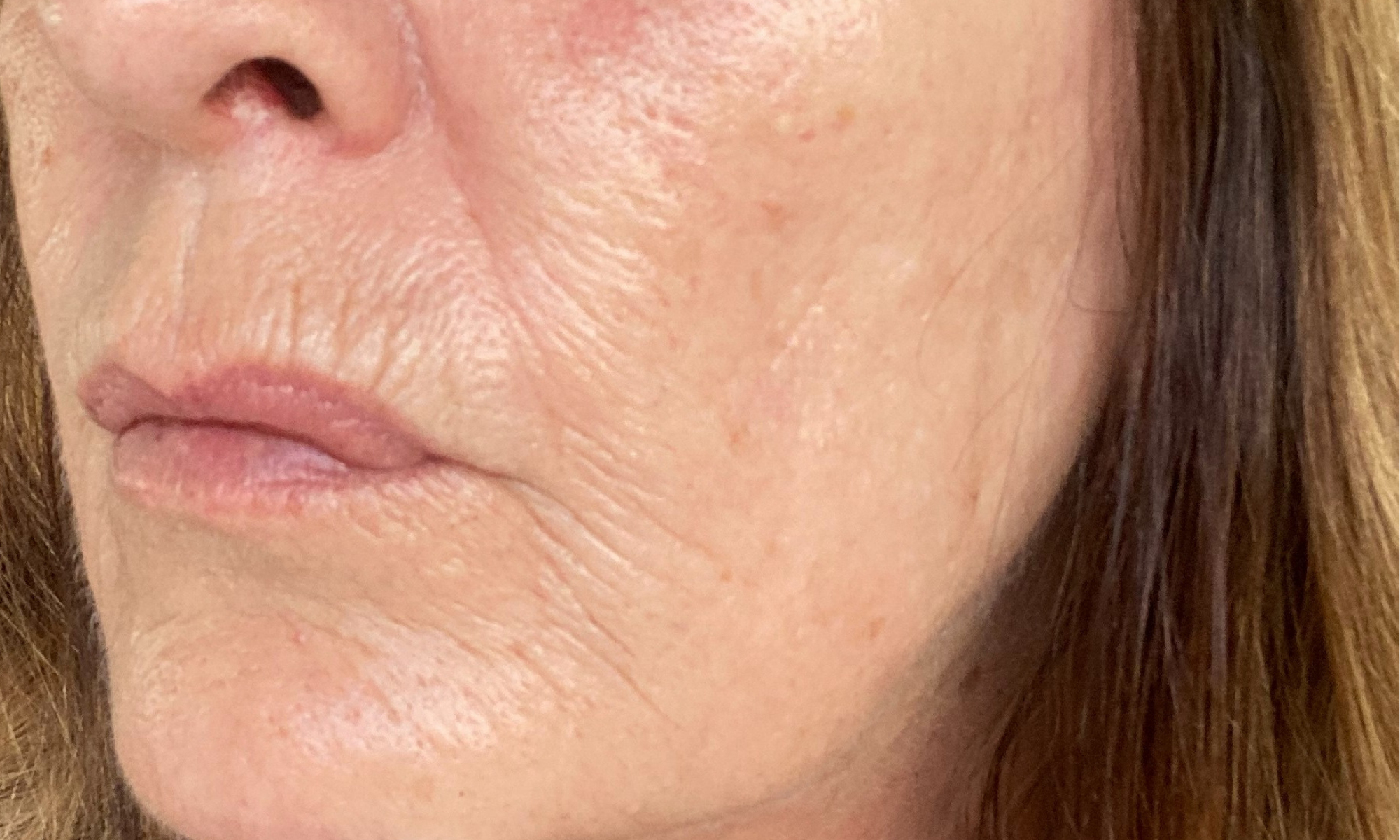
After

WHY DO WE TEST?
Clinical studies are your and our guarantee that the products' effect and tolerability have been examined and found valid by independent dermatologists. Which provides a large degree of certainty that the results are valid
Documented effect means that the products' effect has been examined and found to be valid in clinical studies, carried out by an independent, third-party dermatological testing laboratory.
Dermatological This means that the test results are examined and assessed by dermatologists.
Third-party and independent means that the testing laboratory is not involved in the development or sale of the products, and that their financial earnings are not affected by the outcome of the clinical studies.
In clinical studies on Dr.Ankerstjerne products, the results are based on objective and quantifiable measurements performed by independent dermatologists.

Developed by doctors
Proven effect
Made in Denmark
Clinical studies of Dr.Stine products are conducted by independent dermatologists at a third-party testing laboratory in Poland.
A total of 500 people have participated in the dermatological studies, all of which have been conducted by independent specialists.
The number of participants in each study varies, but we always try to have a sufficient number of participants to obtain statistically significant results.
The testers have received the product in a white sample tube without a logo or name, so they do not know which product or skincare brand they are testing.
The clinical studies have different setups depending on what is being investigated. In the long-term studies, the products are used as the only skin care product, morning and evening, daily for 8 weeks.
The results have been obtained by examining the test subjects' skin with, among other things, advanced 3D skin scanners and other measuring instruments, before and after the test period. No placebo was used. Instead, the results in the effect studies have been obtained by comparing them with the skin care routine that the test subjects used before.
"Skin irritation test" or "Skin tolerability test" is a dermatological test for assessing the skin's tolerance for the product.
Method : The product is applied to the skin with a protective membrane covering it. In this way, the moisture in the product is preserved and the cream stays on the skin without drying out. This reinforces the cream's effect on the skin. The product remains on the skin for 48 hours after which the membrane is removed. The skin is assessed by an independent dermatologist 30 minutes after the occlusion/membrane has been removed and again after 72 hours. If there have been signs of irritation, the test subject's skin is assessed once more after 96 hours. The dermatologist assesses whether there are signs of irritation and looks for, among other things, redness, swelling, bumps, blisters and skin damage. Based on the results, the product is classified as either non-irritating, slightly irritating, moderately irritating and highly irritating.
The test is in accordance with:
- Regulation of the European Parliament and of the Council (EC) No. 1223/2009 of 30
- November 2009 on Cosmetic Products;
- Cosmetics Europe ─ The Personal Care Association (formerly COLIPA) Guidelines: “Product
- Test Guidelines for the Assessment of Human Skin Compatibility 1997”;
- Cosmetics Europe ─ The Personal Care Association (formerly COLIPA) Guidelines for the
- Evaluation of the Efficacy of Cosmetic Products 2008.
WHO IS THE PRODUCT TESTED ON
Below are examples of results for WASH the day off facial cleanser:
- The product has been dermatologically tested on 75 people aged 21 to 69 years.
- 9% (7 out of 75) of the test subjects were men and 91% (68 out of 75) were women.
- 33% of the test subjects have known allergic/atopic (sensitive) skin.
- The test subjects predominantly had skin phototype 2 (Fitzpatrick scale 2)
Result:
All our products have been tested with the "Skin tolerability test".
None of the test subjects showed any signs of an allergic reaction, irritation, redness or swelling, either after 30 minutes or 72 hours. The products are well tolerated and can be classified as non-irritating also for people with atopic/allergic (sensitive) skin.
Tightness and elasticity are biomechanical properties of the skin that are measured using an advanced measuring instrument, Cutometer®. Cutometer® is one of the most recognized and most widely used measuring instruments in dermatology for assessing skin tightness and elasticity. The instrument creates an air suction, i.e. a negative pressure, which the skin is exposed to for a short period. While the skin is exposed to the pressure changes, the instrument's optical parts measure the skin's movements. The skin's resistance to movement when exposed to suction is a measure of skin tightness. The skin's ability to restore its original shape when the suction ends is a measure of the skin's elasticity.
Method:
Test subjects are instructed to stop using other face creams/serums and the like when the test period starts. The test subjects apply the test product to their face twice a day, as the only skin care product, for 8 weeks. The skin's firmness/tightness and elasticity are measured on day 0, and then after 8 weeks. Measurement of the skin's tightness/firmness and elasticity takes place at both times in a room with a temperature of 20±2oC and a humidity of 50±10%, so that the result is not affected by the surroundings. The results of the measurements before and after 8 weeks of using the test product are then compared. In the clinical study, the effect of the test product is compared in this way with the skin care the test person has used in the past.
The test is in accordance with:
- Regulation of the European Parliament and of the Council (EC) No. 1223/2009 of 30
- November 2009 on cosmetic products.
- Cosmetics Europe – The Personal Care Association (previously COLIPA) Guidelines
- "Product Test Guidelines for the Assessment of Human Skin Compatibility 1997."
- Cosmetics Europe – The Personal Care Association (previously COLIPA) Guidelines
- for the Evaluation of the Efficacy of Cosmetic Products 2008.
Wrinkle length and wrinkle depth can be measured with a Visioline® VL 650 measuring instrument. The measuring principle is based on the skin's relief, and thus wrinkles, being replicated using a material called Silflo®. The wrinkles, which normally go down into the skin, will be raised on the replica like a microscopic "mountain" rising up. These "mountains" are illuminated at a special angle so that shadows are formed. The shadows are measurable and visualized using a camera. The measured shadows are used as a basis in mathematical models, so that quantitative measures of wrinkle parameters such as depth and length of wrinkles are obtained.
Method:
Test subjects are instructed to stop using other face creams/serums and the like when the test period starts. The test subjects apply the test product to their face twice a day, as the only skin care product, for 8 weeks. Wrinkle parameters are measured on day 0, and then after 8 weeks. Measurement of the skin's wrinkles takes place at both times in a room with a temperature of 20±2oC and a humidity of 50±10%, so that the result is not affected by the environment. The results of the measurements before and after 8 weeks of using the test product are then compared. In the clinical study, the effect of the test product is compared in this way with the skin care the test person has used in the past.
The test is in accordance with:
- Regulation of the European Parliament and of the Council (EC) No. 1223/2009 of 30
- November 2009 on cosmetic products.
- Cosmetics Europe – The Personal Care Association (previously COLIPA) Guidelines
- "Product Test Guidelines for the Assessment of Human Skin Compatibility 1997."
- Cosmetics Europe – The Personal Care Association (previously COLIPA) Guidelines for the Evaluation of the Efficacy of Cosmetic Products 2008.
STUDIES
The study complies with several guidelines and regulations, including:
- European Parliament and Council Regulation (EC) No. 1223/2009 on cosmetic products.
- Cosmetics Europe Guidelines for Human Skin Compatibility (1997), Efficacy of Cosmetic Products (2008), and Product Claim Substantiation (2019).
Investigational Product:
- Product Reference: DR. ANKERSTJERNE ANTI-AGE NIGHTCREAM
- Intended Use: Skin care
- Method of Use: Apply to face in the morning and before bedtime.
- Contraindications: Acute inflammation requiring pharmacological treatment and allergy or hypersensitivity to any ingredients.
Study Description:
- Aim: To confirm or exclude the declared efficiency of the product.
- Duration: 42 days (+/- 2 days)
Testing Methodology:
Instrumental Test of Skin Firmness and Elasticity (Cutometer® MPA 580):
- Objective: To measure the impact of the product on skin firmness and elasticity.
- Subjects: 27 women aged 35-65 with normal, dry, sensitive and non-sensitive skin types.
- Procedure: Measurements before product application (D0) and after 42 days (D42) of regular use.
- Environment: Air-conditioned room at 20±2°C and relative humidity 50±10%.
Wrinkles Analysis (Primos 3D Lite):
- Objective: To assess wrinkle length, depth, count, volume, and area.
- Procedure: Measurements before product application (D0) and after 42 days (D42) of regular use.
- Environment: Air-conditioned room at 20±2°C and relative humidity 50±10%.
- Statistical Analysis:
- Performed using STATISTICA 13.
- Paired sample T test or Wilcoxon signed rank test was used.
- Significance level set at p<0.05.
Results:
- Skin Firmness and Elasticity:
- Elasticity Improvement: 96% of subjects showed improvement with an average increase of 9% (statistically significant).
- Firmness Improvement: 100% of subjects showed improvement with an average decrease of 15% in the parameter value (statistically significant).
- Wrinkles Analysis:
- Length Reduction: 88% of subjects showed a decrease with an average reduction of 4% (statistically significant).
- Wrinkle Count Reduction: 92% of subjects showed a decrease with an average reduction of 8% (statistically significant).
- Volume Reduction: 80% of subjects showed a decrease with an average reduction of 6% (statistically significant).
- Wrinkle Area Reduction: 88% of subjects showed a decrease with an average reduction of 3% (statistically significant).
Conclusion:
After 42 days of regular use, the DR.ANKERSTJERNE ANTI-AGE NIGHTCREAM:
- Improves Skin Elasticity and Firmness: Statistically significant improvements.
- Reduces Wrinkles Length and Depth: Statistically significant reduction in length.
- Reduces Wrinkle Count, Volume, and Area: Statistically significant reductions.
This report confirms the efficacy of the DR.ANKERSTJERNE ANTI-AGE NIGHTCREAM based on instrumental tests.
The study adhered to several regulatory and guideline frameworks, including:
- European Parliament and Council Regulation (EC) No. 1223/2009 on cosmetic products.
- Guidelines from Cosmetics Europe (formerly COLIPA).
Investigational Product
- Products Tested : DR.ANKERSTJERNE SUPERFOOD MULTIPURPOSE VITAMIN FACEGEL and DR.ANKERSTJERNE URBAN PROTECTION DAYCREAM.
- Intended Use : Skin care.
- Method of Use : First apply the face gel, followed by the day cream after 1-2 minutes.
Study Description
- Aim : To confirm or exclude the declared efficiency of the products.
- Duration : 42 days (+/- 2 days).
- Subjects : 52 women aged 35-65 with normal, dry, non-sensitive skin.
Testing Methodology
Instrumental Test of Skin Elasticity and Firmness :
- Measured using Cutometer® MPA 580.
- Conducted on 52 subjects, measurements taken before (D0) and after 42 days (D42) of regular use.
- Conducted in controlled conditions (20±2°C, relative humidity 50±10%).
Wrinkle Analysis :
- Measured using Primo's 3D Lite.
- Evaluated wrinkle length, depth, count, volume, and area.
- Conducted on 52 subjects, measurements taken before (D0) and after 42 days (D42) of regular use.
- Statistical analysis :
- Used STATISTICA 13, employing Paired sample T test or Wilcoxon signed rank test.
- Significance level set at p<0.05.
Results
Skin Elasticity :
- Statistically significant improvement (p-value = 0.0000).
- 100% of subjects showed a positive effect.
Skin Firmness :
- Statistically significant improvement (p-value = 0.0000).
- 96% of subjects showed a positive effect.
Wrinkle Length :
- Statistically significant reduction (p-value = 0.0000).
- 78% of subjects showed a positive effect.
Wrinkle Depth :
- Statistically significant reduction (p-value = 0.0054).
- 67% of subjects showed a positive effect.
Wrinkle Count :
- Statistically significant reduction (p-value = 0.0000).
- 96% of subjects showed a positive effect.
Wrinkle Volume :
- Statistically significant reduction (p-value = 0.0002).
- 69% of subjects showed a positive effect.
- Wrinkle Area :
- Statistically significant reduction (p-value = 0.0000).
- 84% of subjects showed a positive effect.
Conclusion
Under the study conditions, after 42 days of regular use, the products DR.ANKERSTJERNE SUPERFOOD MULTIPURPOSE VITAMIN FACEGEL and DR.ANKERSTJERNE URBAN PROTECTION DAYCREAM:
- Significantly improved skin elasticity by an average of 11%.
- Significantly improved skin firmness by an average of 13%.
- Reduced wrinkle count by an average of 9%.
- Significantly reduced wrinkle length, depth, volume and wrinkle area
These results confirm the efficacy of the products in enhancing skin biomechanical properties and reducing various aspects of wrinkles. The detailed results, methodology, and compliance ensure the reliability and validity of the findings, supporting the claimed benefits of the investigated skin care products.